Hello, welcome to Hangzhou Zhengda Medical Co., Ltd.
NEWS CENTER
PRODUCTS CLASS
-
2026-2-10 hits:475
-
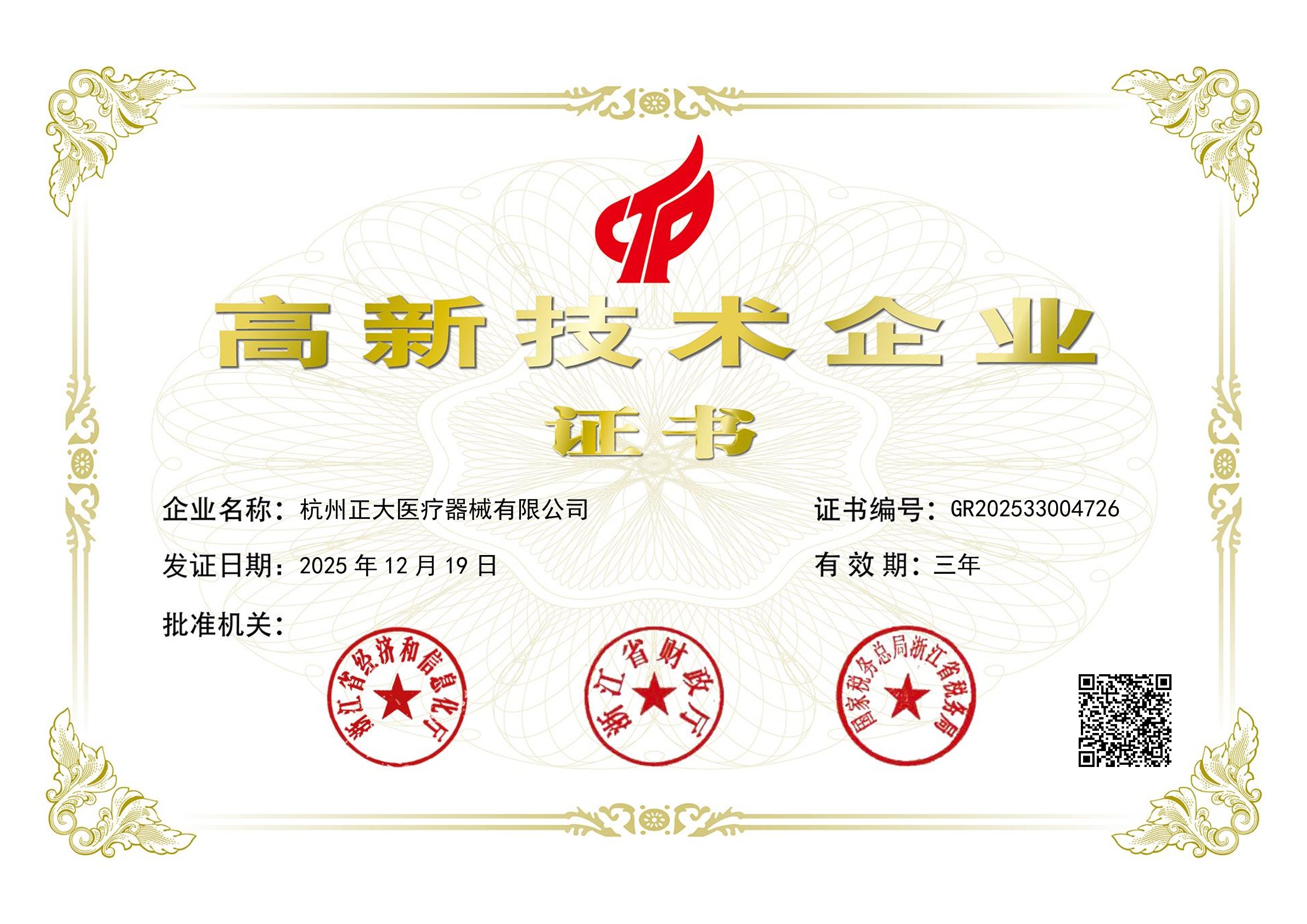
Revalidated Innovation: Hangzhou Zhengda Re-certified as High-Tech Enterprise2026-1-20 hits:967
-
MEDICA 2025 Concludes Successfully | The exhibition has concluded successfully with fruitful results.2025-11-21 hits:2978Our company showcased R&D, manufacturing and system capabilities at MEDICA 2025, gaining meaningful insights into global medical technology development.
-
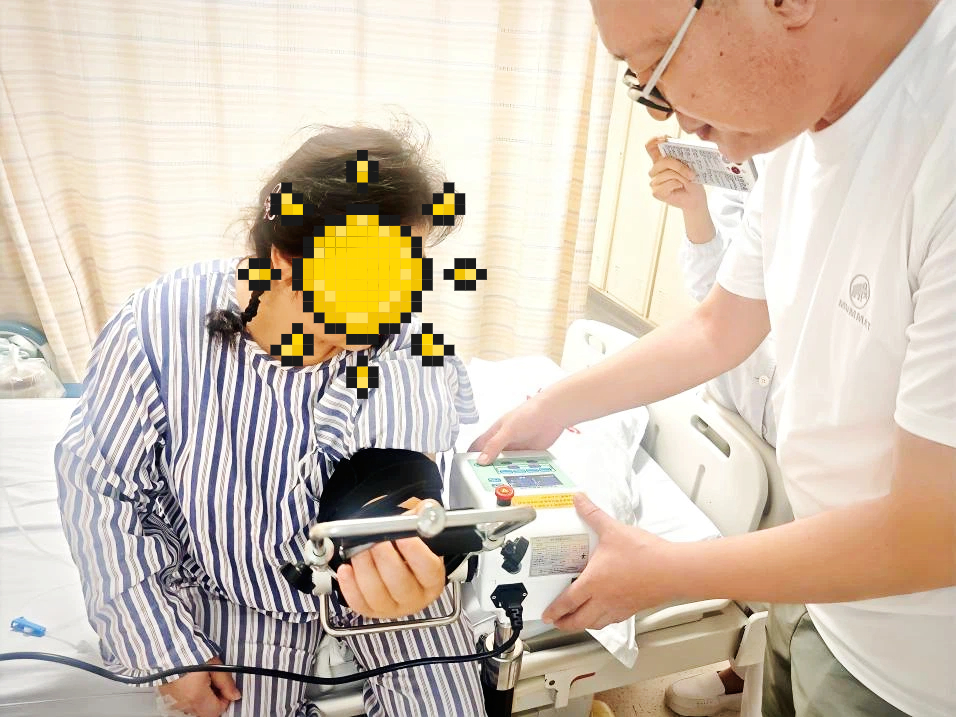
Engineers Visit a Hospital in Jiangsu | Elbow Joint Rehabilitation Device Makes Postoperative Rehabilitation More Scientific2025-11-6 hits:3391Hangzhou Zhengda Medical provided elbow joint CPM training at a hospital in Jiangsu, introducing intelligent rehabilitation devices that support scientific and efficient postoperative recovery.